How does the reaction occur?
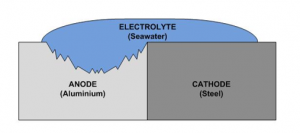
The catalyst for the corrosion is the electrode potential that exists between the two metals. The continuous flow of electrons from one metal to another fuels the corrosive process. This potential difference causes electrons to migrate from the more anodic metal to the more cathodic metal.
The hungry “cathodic” material literally eats away at the seemingly weaker “anodic” material.
To make this relevant to what we do as a balustrading company the electrolyte is water, the more conductive the electrolyte the higher the risk of corrosion, so for us installing near the seaside will pose a higher risk than an install in central London, due to the higher salt content in the water in the atmosphere. The image below demonstrates how the corrosion occurs when all of the ingredients come together without suitable isolation from each other.
How does it affect our balustrade systems?
We install various types of materials together that are exposed to the elements on a daily basis. However it’s important to remember that without the electrolyte no corrosion will occur, so if the materials are in contact in a dry environment they are not affected.
The catalyst can still be present, however, inside a building. The high humidity of a leisure centre can be enough of an electrolyte in some situations so it would be prudent to isolate the materials.
The list below are a selection of metals we use for our products starting with the most noble down to the least noble:
- Stainless Steel 316 – CATHODIC (most noble)
- Stainless Steel 304
- Copper alloys (brass, bronze)
- Mild steel
- Aluminium
- Zinc (hot-dip, die cast, or plated) – ANODIC (least noble and therefore more susceptible to corrosion).
To prevent corrosion we need to keep the materials isolated from each other. We provide the solution to this through powder coatings and gasket materials between the metals to provide electrical insulation.
We often get questioned about galvanic corrosion between the metals of our fixings and products. As an example, corrosion is very unlikely when using stainless steel fixings tight onto a lesser noble material such as aluminium, this is due to the contact area being such a small percentage of the aluminium, the ratio between the two would be too great for galvanic corrosion to occur.
For more details on this please contact Darryl Snipe on darryl.snipe@basystems.co.uk
Back to blog